How Does Thermal Energy Relate To Temperature During Condensation
In general,condensation is the modify of the physical state of matter from the gas phase into the liquid stage and is the reverse of vaporization. Catamenia processes associated with condensation on a solid surface are almost a mirror image of those involved in boiling.
In general, three singled-out forms of condensation are observed:
- Film condensation
- Dropwise condensation
- Direct contact condensation
Similarly, as in the previous chapter, in this chapter, nosotros volition discuss oestrus transfer with stage change, but in this case, we will discuss condensation of gas-phase (vapor-to-liquid stage change).
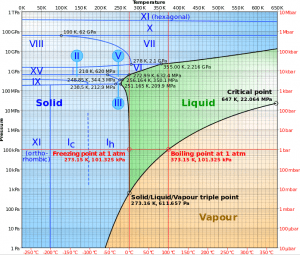
In general, condensation is the modify of the physical state of thing from the gas stage into the liquid stage and is the reverse of vaporization. Flow processes associated with condensation on a solid surface are about a mirror image of those involved in humid. Condensation occurs when the temperature of the vapor is reduced below its saturation temperature or when the vapor pressure increases above its saturation parameters (see phase diagram of water).
Source: wikipedia.org CC Past-SA
For condensation, latent heat effects associated with the phase change are pregnant, similar to boiling, merely reverse. Notation that the enthalpy of condensation (or oestrus of condensation) is by definition equal to the enthalpy of vaporization with the opposite sign. Latent heat is the amount of heat added to or removed from a substance to produce a phase change. This energy breaks down the intermolecular attractive forces during vaporization and must provide the energy necessary to expand the gas (the pΔV piece of work). When latent heat is added or removed, no temperature change occurs. The enthalpy of vaporization is a office of the pressure level at which that transformation takes identify.

Latent oestrus of condensation – water at 0.one MPa (atmospheric force per unit area)
h lg = – 2257 kJ/kg
Latent heat of condensation – water at 3 MPa
h lg = – 1795 kJ/kg
Latent rut of condensation – water at 16 MPa (pressure inside a pressurizer)
h lg = – 931 kJ/kg
The heat of condensation diminishes with increasing pressure while the boiling indicate increases, and it vanishes completely at a sure point called the critical point. Above the disquisitional point, the liquid and vapor phases are duplicate, and the substance is called a supercritical fluid.
The change from the liquid to the vapor state due to boiling is sustained by estrus transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very loftier for common pressures. Therefore large amounts of heat can be transferred during boiling and condensation at a abiding temperature. Oestrus transfer coefficients, h, associated with boiling and condensation are typically much higher than those encountered in other forms of convection processes that involve a unmarried phase.
Condensation Modes – Types of Condensation
From a practical technology point of view, condensation tin be categorized according to several criteria.
In general, 3 distinct forms of condensation are observed:
- Film condensation. In picture show condensation, the condensate wets the surface and forms a liquid film that slides down under the influence of gravity. Film condensation results in low heat transfer rates as the picture show condensate impedes the oestrus transfer. The thickness of the film formed depends on many parameters, including orientation of the surface, viscosity, rate of condensation, etc. The film increases the thermal resistance to heat catamenia between the surface and the vapor, and the oestrus transfer rate is reduced because of this resistance.
- Dropwise condensation. The condensed vapor forms droplets on the surface instead of a continuous film in dropwise condensation. Dropwise condensation can occur when the surface is nonwetting, or these droplets are taken away from the surface by external catamenia or gravity. The vapor is in direct contact with the surface over most of the expanse, and heat transfer rates are much higher (more than 3 – 10 times higher) as at that place is very fiddling resistance for estrus flow betwixt the vapor and the surface. The aerosol develop at nucleation sites (points of surface imperfections such as pit, scratch, and cavities) and grow in size as more vapor condenses on its exposed surface. For steam condensers, it is common practice to use surface coatings (silicons, Teflon, or waxes) that inhibit wetting and stimulate dropwise condensation. But coatings gradually lose their effectiveness due to removal, oxidation, etc. In practice, no surface is establish to continue as nonwetting over any length of fourth dimension. Since it is oftentimes difficult to maintain this condition in most engineering applications, using the value of rut transfer coefficients assuming dropwise condensation for design purposes is not advisable.
- Direct contact condensation. DCC, straight contact condensation occurs when vapor is brought into contact with a cold liquid. As in jet condensers, the cooling h2o is sprayed on the frazzle steam, and there is direct contact between the exhaust steam and cooling water. The condensation process is very fast and efficient, simply cooling h2o and condensed steam are mixed upwards hither. The advantages of direct contact condensation over the conventional processes using metallic transfer surfaces are due to the relative simplicity of design, less corrosion and scaling problems, lower maintenance costs, higher specific transfer areas, and higher transfer rates. Despite these advantages, jet condensers are not usual in thermal power plants, especially due to the loss of condensate.



Condensation in Power Plants – Main Condensator
 Themaster steam condenser (MC) arrangement is designed tocondense anddeaerate the exhaust steam from the main turbine and provide a heat sink for the turbine featherbed system. The exhausted steam from the LP turbines is condensed by passing over tubes containing water from the cooling system. There is a main condenser unit nether each LP turbine, usually below the turbine, with its axis perpendicular to the turbine axis. Since nuclear power plants normally contain an auxiliary condenser (e.chiliad., to condense steam from steam-driven feedwater pumps), engineers utilise a "main condenser ".
Themaster steam condenser (MC) arrangement is designed tocondense anddeaerate the exhaust steam from the main turbine and provide a heat sink for the turbine featherbed system. The exhausted steam from the LP turbines is condensed by passing over tubes containing water from the cooling system. There is a main condenser unit nether each LP turbine, usually below the turbine, with its axis perpendicular to the turbine axis. Since nuclear power plants normally contain an auxiliary condenser (e.chiliad., to condense steam from steam-driven feedwater pumps), engineers utilise a "main condenser ".
Come across besides: Main Condenser
The condenser must maintain a sufficient depression vacuum to increase the ability plant efficiency. The vacuum pumps maintain a sufficient vacuum in the condenser by extracting air and uncondensed gases. The lowest feasible condenser pressure is the saturation pressure corresponding to the ambient temperature (e.k., the absolute pressure of0.008 MPa,which corresponds to 41.5°C). Notation that there is e'er a temperature difference between (effectually ΔT = 14°C) the condenser temperature and the ambient temperature, which originates from condensers' finite size and efficiency. Since neither the condenser is a 100% efficient heat exchanger, in that location is always a temperature divergence betwixt the saturation temperature (secondary side) and the coolant temperature in the cooling system. Moreover, there is design inefficiency, which decreases the overall efficiency of the turbine. Ideally, the steam wearied into the condenser would take no subcooling. But existent condensers are designed to subcool the liquid by a few degrees Celsius to avert the suction cavitation in the condensate pumps.
Thesteam condensers are broadly classified into 2 types:
- Surface condensers (or non-mixing type condensers). There is no direct contact between the frazzle steam and the cooling h2o in surface condensers.
- Jet condensers (or mixing type condensers). At that place is directly contact between the exhaust steam and cooling water in jet condensers.
Condensation in Pressurizer

Apressurizer is a component of a pressurized h2o reactor. Pressure in the primary circuitof PWRs is maintained by apressurizer, a separate vessel connected to the principal circuit (hot leg), and partially filled with water heated to the saturation temperature (humid point) for the desired force per unit area by submergedelectric heaters. The temperature in the pressurizer tin can be maintained at 350 °C (662 °F), which gives a subcooling margin (the deviation betwixt the pressurizer temperature and the highest temperature in the reactor core) of 30 °C. Subcooling margin is a very of import prophylactic parameter of PWRs since the humid in the reactor core must be excluded. The bones blueprint of the pressurized water reactorincludes such a requirement that the coolant (water) in the reactor coolant organization must not boil. To achieve this, the coolant in the reactor coolant system is maintained at a pressure level sufficiently high that humid does non occur at the coolant temperatures experienced while the plant is operating or in an analyzed transient.
Functions
Pressure in the pressurizer is controlled past varying the temperature of the coolant in the pressurizer. For these purposes, two systems are installed. H2o spray organization andelectrical heaters organisation. The volume of the pressurizer (tens of cubic meters) is filled with water on saturation parameters and steam. The h2o spray system (relatively cool water – from the cold leg) tin can decrease the force per unit area in the vessel by condensing the steamon water aerosol sprayed in the vessel. Since vapor is brought into contact with a common cold liquid, in this case, nosotros are talking about direct contact condensation. On the other mitt, the submerged electrical heaters are designed to increase the pressure past evaporating the water in the vessel. Water pressure in a closed organization tracks water temperature straight; as the temperature rises, the pressure goes up.
Condensation in Containment Building
In case of Design Footing Accidents such every bit the Big Break Loss of Coolant Blow (LBLOCA), the pressure level increase is usually significant, and agile containment systems (pressure level-suppression systems) must be available to maintain the integrity (to keep the pressure and temperature under certain limits) of the containment building.
Force per unit area-suppression systems are critical to safety and greatly affect the size of containment. Suppression refers tocondensing the steam afterward a major break has released it from the cooling system. There are many designs of suppression systems effectually the world.
Virtually Pressurized H2o Reactors (PWRs) containments use 2-stage pressure level-suppression systems:
- Fan Libation System. This organisation circulates air through heat exchangers and filters to provide the cooling of containment temper. Since this system is not sufficient for suppression during the astringent loss of coolant accidents, the containment spray system must be available equally the secondary active force per unit area-suppression system.
- Containment Spray System. This system consist usually of three elements:
- Spray Organisation Pump
- Spray System Tank
- Spray System Rings and Nozzles
When pressure increases inside the containment are indicated, the containment spray system is automatically started, and the pumps (usually with 3×100% redundancy) take suction from the tank (refueling h2o storage tank tin too be used) and pump the water into spray nozzles located in the upper function of the containment. The water droplets, being cooler than the steam, will remove rut from the steam, which will cause the steam to condense. This volition cause a reduction in the pressure of the edifice and will also reduce the temperature of the containment atmosphere. The spray arrangement usually contains extra chemical additives dissolved in the tank to enhance the removal of particular radionuclides from the containment temper. Specially radioiodine, which is of particular importance, tin be finer bonded to potassium hydroxide or sodium hydroxide.
Nearly Boiling H2o Reactors (BWR) containments use pressure-suppression pools to maintain the integrity of the containment building. The major containment designs are Marking I, Mark Two, and Marker Iii. The Marking I and Marking Two containments consist of ii master parts:
- Drywell. A drywell houses the reactor coolant system.
- Wetwell. A wetwell is a suppression chamber that stores a large bounding main, and therefore, it is normally called the suppression pool.
H2o spray systems are normally installed in both the drywell and the wetwell. The Mark III design consists of chief containment and a drywell.
Containment buildings and containment force per unit area-suppression systems vary widely depending on certain reactor designs. In some cases, unique technologies can be installed. For example, the containment building of Loviisa NPP uses two water ice condensers as the pressure-suppression arrangement.
References:
Heat Transfer:
- Fundamentals of Rut and Mass Transfer, 7th Edition. Theodore L. Bergman, Adrienne S. Lavine, Frank P. Incropera. John Wiley & Sons, Incorporated, 2011. ISBN: 9781118137253.
- Heat and Mass Transfer. Yunus A. Cengel. McGraw-Hill Education, 2011. ISBN: 9780071077866.
- U.S. Department of Energy, Thermodynamics, Heat Transfer and Fluid Menses. DOE Fundamentals Handbook, Volume two of 3. May 2016.
Nuclear and Reactor Physics:
- J. R. Lamarsh, Introduction to Nuclear Reactor Theory, 2nd ed., Addison-Wesley, Reading, MA (1983).
- J. R. Lamarsh, A. J. Baratta, Introduction to Nuclear Engineering, 3d ed., Prentice-Hall, 2001, ISBN: 0-201-82498-1.
- Due west. One thousand. Stacey, Nuclear Reactor Physics, John Wiley & Sons, 2001, ISBN: 0- 471-39127-1.
- Glasstone, Sesonske. Nuclear Reactor Technology: Reactor Systems Engineering science, Springer; 4th edition, 1994, ISBN: 978-0412985317
- W.Due south.C. Williams. Nuclear and Particle Physics. Clarendon Press; one edition, 1991, ISBN: 978-0198520467
- 1000.R.Keepin. Physics of Nuclear Kinetics. Addison-Wesley Pub. Co; 1st edition, 1965
- Robert Reed Burn, Introduction to Nuclear Reactor Operation, 1988.
- U.S. Department of Energy, Nuclear Physics and Reactor Theory. DOE Fundamentals Handbook, Volume 1 and two. January 1993.
- Paul Reuss, Neutron Physics. EDP Sciences, 2008. ISBN: 978-2759800414.
Advanced Reactor Physics:
- K. O. Ott, W. A. Bezella, Introductory Nuclear Reactor Statics, American Nuclear Order, Revised edition (1989), 1989, ISBN: 0-894-48033-2.
- G. O. Ott, R. J. Neuhold, Introductory Nuclear Reactor Dynamics, American Nuclear Society, 1985, ISBN: 0-894-48029-iv.
- D. Fifty. Hetrick, Dynamics of Nuclear Reactors, American Nuclear Lodge, 1993, ISBN: 0-894-48453-2.
- E. East. Lewis, W. F. Miller, Computational Methods of Neutron Transport, American Nuclear Society, 1993, ISBN: 0-894-48452-4.
See higher up:
Boiling and Condensation
Source: https://www.nuclear-power.com/nuclear-engineering/heat-transfer/boiling-and-condensation/condensation/

 By definition,multiphase catamenia is the interactive period oftwo or moredistinct phases with common interfaces in, say, a conduit. Each stage, representing a book fraction (or mass fraction) of solid, liquid, or gaseous matter, has its ain backdrop,velocity, andtemperature.
By definition,multiphase catamenia is the interactive period oftwo or moredistinct phases with common interfaces in, say, a conduit. Each stage, representing a book fraction (or mass fraction) of solid, liquid, or gaseous matter, has its ain backdrop,velocity, andtemperature.
0 Response to "How Does Thermal Energy Relate To Temperature During Condensation"
Post a Comment